· 8 min read
Project for Research Platform
Specialized Upgrade Project for Research Platform's Underlying Data Middleware Adaptation to Enhance Search Accuracy.
Project and Content Description
Project Name: Specialized Upgrade Project for Research Platform’s Underlying Data Middleware Adaptation to Enhance Search Accuracy
Project Background: The project has undergone 5 iterative versions, deployed across multiple hospital sites. However, ongoing maintenance revealed systemic issues:
I. Core Operational Challenges
- System Performance
- Slow search responses
- Incomplete data exports
- Cumbersome project creation workflows
- Variable Generation Management
- Unclear and chaotic underlying variable generation steps
- Difficulties in monitoring workflows
- Design Inconsistency
- Lack of unified design language
- Excessive redundant content
II. Product-Market Mismatch
- Expectation Gap
- Strong marketing appeal and user recognition of product concepts
- Significant discrepancies between promised features and actual user experience
- Competitive Disadvantage
- Requirement for high-cost customizations to match competitor benchmarks
- Low ROI leading to sales team reluctance in pursuing deals
- Market intelligence deficiencies regarding key competitors
Project Objectives
- Competitiveness Enhancement
- Resolve existing system flaws through actionable solutions
- Feature Parity
- Close functional and parametric gaps against competitors
- Differentiated Value Proposition
- Execute holistic product upgrades to establish unique market positioning
Project Analysis
I. Core Functional Issues
Slow Search Performance
- Example: Query “Patients diagnosed with congenital heart disease (CHD) from 2015 to present with white blood cell count ≥2” returned 22,345 patients in 1 min 42 sec on our system.
- Competitor Benchmark: Similar query took ~1 min 10 sec for 20,344 patients.
Incomplete Search Results
- Critical Gap: Standardized queries fail to capture synonym variations (e.g., “CHD” = “Congenital Heart Defect” | “Congenital Cardiovascular Anomaly”).
- Impact: Users must manually combine multiple diagnostic definitions.
Abnormal Data Export Formats
- Key Issues:
- Column mismatches preventing direct import into SPSS/R
- Truncated datasets requiring repeated export requests
- Mandatory security approvals delaying data access
- Key Issues:
Subproject Creation Challenges
- Pain Points:
- Complex inclusion/exclusion criteria adjustment
- Statistically demanding design processes
- Low user adoption due to steep learning curve
- Pain Points:
Missing Custom Query Capabilities
- Critical Unmet Need: Inability to implement:
- Search-triggered events
- Variable-driven workflows
- Custom extraction rules
- Critical Unmet Need: Inability to implement:
II. Architectural Deficiencies
Fragmented Product Architecture
- Problem: Isolated modules (Platform Management / Specialty Disease DB / Infrastructure Settings) with:
- Non-inheritable configurations
- Duplicated search functions (e.g., specialty DB variables require separate permission sets)
- Cost Impact: High maintenance and customization expenses
- Problem: Isolated modules (Platform Management / Specialty Disease DB / Infrastructure Settings) with:
Inconsistent Design Language
- UX Failures:
- Disjointed interaction patterns forcing constant context switching
- Incoherent modal dialog behaviors
- UX Failures:
Chaotic Permission Design
- Example: Users with specialty DB access still require additional approvals for:
- Cross-database searches
- Subproject creation
- Data exports
- Example: Users with specialty DB access still require additional approvals for:
Third-Party Variable Generation Dependency
- Systemic Risk:
- Unauditable core logic in variable management
- Frequent ID binding failures and undetected data loss
- Systemic Risk:
Project Plan
Vision: Productize solutions through component-based development to resolve core issues, bridge functional gaps, and establish competitive differentiation.
Key Deliverables:
- Business Scope Segmentation
- Map high-impact scenarios (e.g., complex cohort searches)
- Priority-Driven Sprint Planning
- Assign cross-functional teams to:
- Search engine optimization
- Unified variable generation workflow
- Permission architecture redesign
- Assign cross-functional teams to:
- Modular Development
- Build reusable components (e.g., standardized export adapter)
- Integrated Functional Testing
- Validate end-to-end workflows (search → export → analysis)
- Pre-release Validation
- Simulate real-world usage scripts
- Controlled Pilot Deployment
- Target 3 hospital sites for phased rollout
- Structured Training Program
- Role-based certification paths
- Performance Monitoring Framework
- Track:
- Query response latency
- Export success rate
- User adoption metrics
- Track:
Functional Specification Details
| Tier 1 Function | Tier 2 Function | Functional Description |
|---|---|---|
| Data Integration | Data Acquisition | Connects multiple data warehouses; collects multi-source data; supports custom processing and data masking |
| Data Cleansing | Standardizes data using EMPI (Enterprise Master Patient Index) and MDM (Master Data Management) technologies | |
| Data Quality Control | Verifies data sources/records; enforces rule-based quality checks for validity, standardization, and anonymization | |
| Visual Operations | Provides integrated monitoring dashboard for data integration, collection, and quality control processes | |
| Specialty DB Management | Specialty DB Configuration | Defines disease criteria, inclusion/exclusion standards, subscription rules, disease tags, and cohort debugging |
| Variable Generation | Configures domain-specific variables: data sources, binding logic, input methods, display formats, synchronization, and custom calculations | |
| Specialty DB Dashboard | Visualizes patient details, cohort inclusion status, core variable distributions, patient groupings, and follow-up/data filling progress | |
| Data Warehouse | Displays raw collected datasets in tabular/form views; allows authorized users to edit source data | |
| Project & Search | Enables authorized users to search/export data, and create subprojects | |
| Data QC Management | Implements rule-based quality control: variable-level checks, security-tiered alerts, and audit trails | |
| Data Collection Assistant | Automates variable-based data collection; notifies clinicians; displays collection details for verification | |
| Disease Follow-up | Configures follow-up content, schedules, reminders, and closure policies | |
| Specialty Settings | Manages permissions, roles, anonymization, audit logs, effective dates; controls functional access (e.g., export/view) | |
| Platform Management | Advanced Search | Supports full-text/patient/conditional/event-based searches; multithreaded search; export capabilities |
| Subproject Management | Manages patient inclusion/exclusion, favorites, patient lists, custom follow-up plans, mobile forms, data policies, logs, anonymization. Supports prospective/retrospective studies. | |
| Research Perspective View | Displays all integrated patient data in a unified patient-centric view | |
| Data Exploration | Enables in-dataset cleansing (missing value imputation, text filtering, variable binning/merging/derivation), dataset versioning, variable/event management, and statistical analysis (descriptive/differential tests) | |
| Platform Settings | Configures platform permissions, roles, anonymization, audit logs, external access, organizations, effective dates; controls functional access |
Notes: Key Terminology Rationale
| Chinese Term | English Translation | Justification |
|---|---|---|
| 数据脱敏 | Data Masking | Industry-standard privacy protection term |
| 纳排标准 | Inclusion/Exclusion Standards | Clinical research convention |
| 变量生产 | Variable Generation | Consistent with prior system terminology |
| 随访 | Follow-up | Medical domain standard |
| 多线程搜索 | Multithreaded Search | Technical accuracy for parallel processing |
| 前瞻性/回顾性研究 | Prospective/Retrospective Studies | Research methodology standards |
| 数据质控 | Data Quality Control (QC) | Maintains consistency across modules |
| 科研视图 | Research Perspective View | Emphasizes clinical research context over literal “scientific view” |
| 变量衍生计算 | Derived Variable Calculations | Precise description of computational logic |
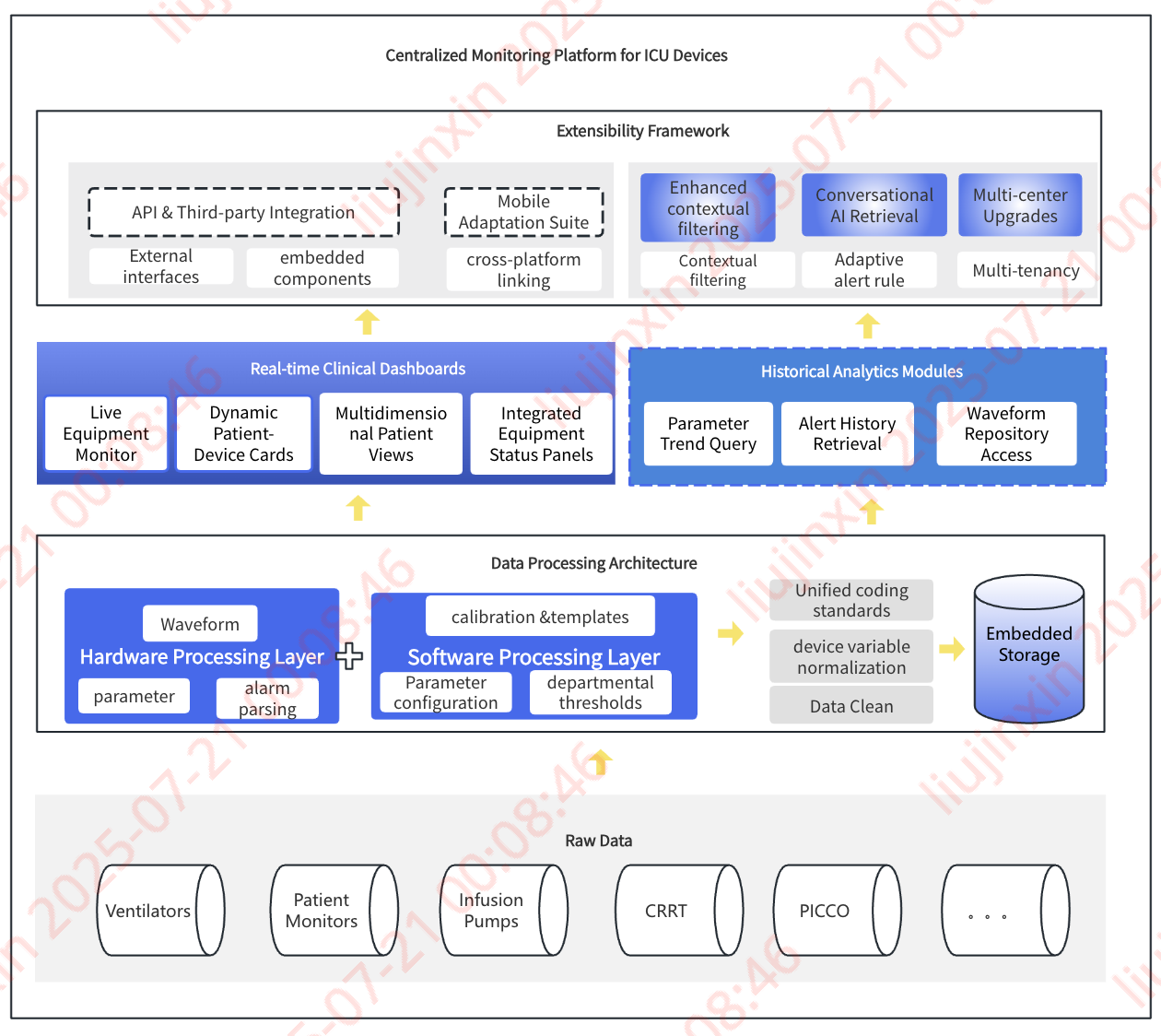
Simplified Data Cleansing Workflow
I. Process Overview
Illustrative workflow for integrating foundational hospital data sources (HIS, LIS, PACS, CIS): 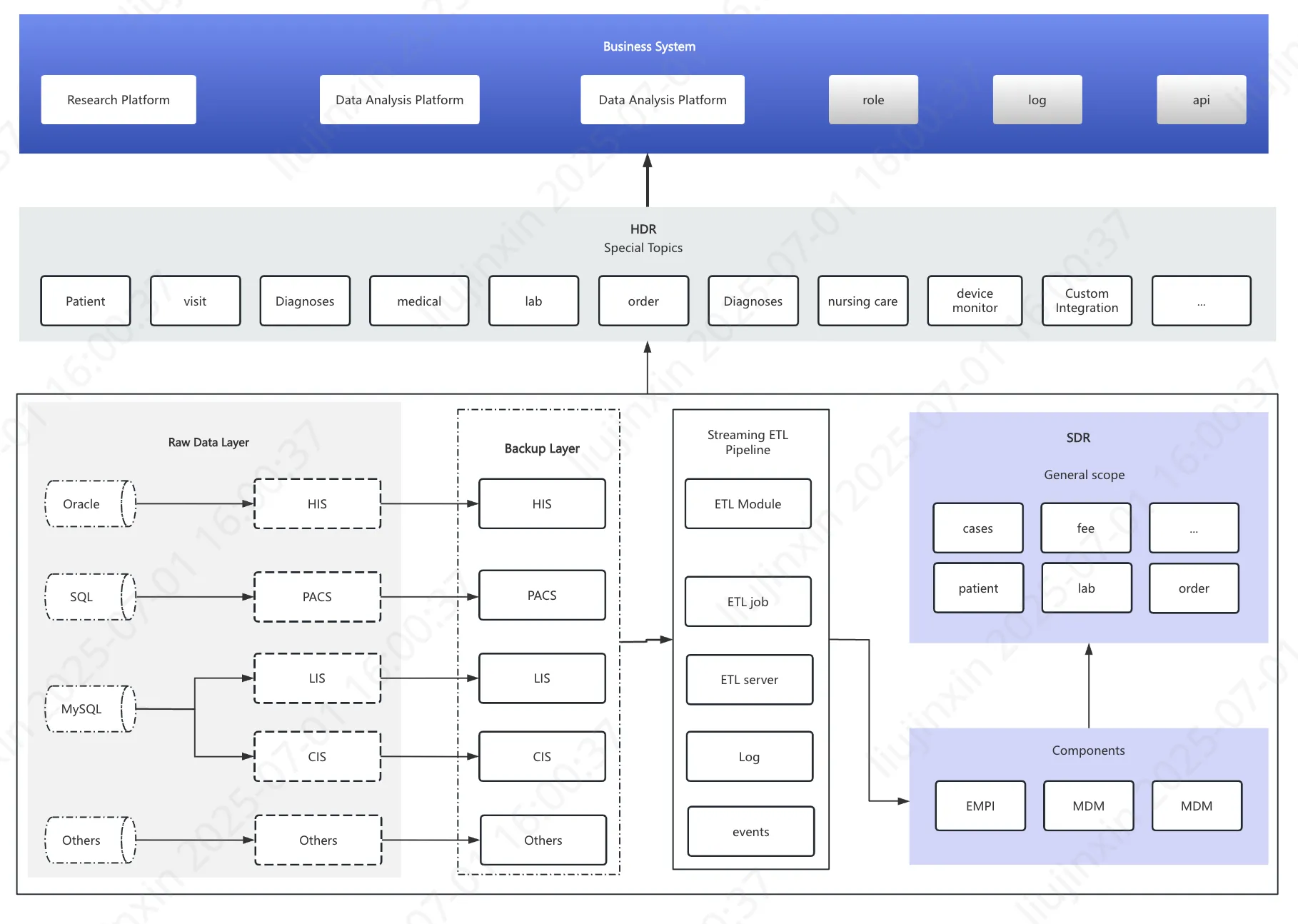 Step 1: Multi-Database Adaptation → Extract backup mirror repositories from source databases
Step 1: Multi-Database Adaptation → Extract backup mirror repositories from source databases
Step 2: ETL Pipeline Execution → Run scheduled ETL jobs with embedded:
- Data validation events
- Quality control rules
- Script-based monitoring
Step 3: Standardization Components → Apply integrated modules:
- EMPI: Auto-correct patient IDs
- MDM: Normalize dictionary terms
- Knowledge Graph: Enable future AI queries
Step 4: SDR (Source Data Repository) → Generate enterprise-level reusable dataset → Supports multi-site table/field sharing
Step 5: HDR (Harmonized Data Repository) → Create enhanced dataset with:
- Custom domain extractions
- On-demand variable generation
- Example transformation:
-- Convert clinical values to variables
CREATE VARIABLE @is_CHD AS
SELECT patient_id,
CASE WHEN diagnosis_code IN ('Q20-Q28') THEN 1 ELSE 0 END
FROM clinical_records
-- Scheduled daily batch processingStep 6: Utilization Paths → HDR powers:
- Hospital-wide queries
- Operational dashboards
- Direct feeding of Specialty DB domains
II. Key Innovations
1. Traceability Mechanism → Preserves original record IDs + standardization metadata → Visualizes processing workflow with audit trails
2. Standardized Componentization → Maintains consistency through:
- Scheduled MDM dictionary updates
- Configurable EMPI rules
3. Breakthrough HDR-to-Variable Conversion → Implements:
- Encapsulated computation logic (hidden backend scripts)
- Transparent value derivation (user-visible source data & transformation details)
- Enables precision in:
- Search result verification
- Export auditing
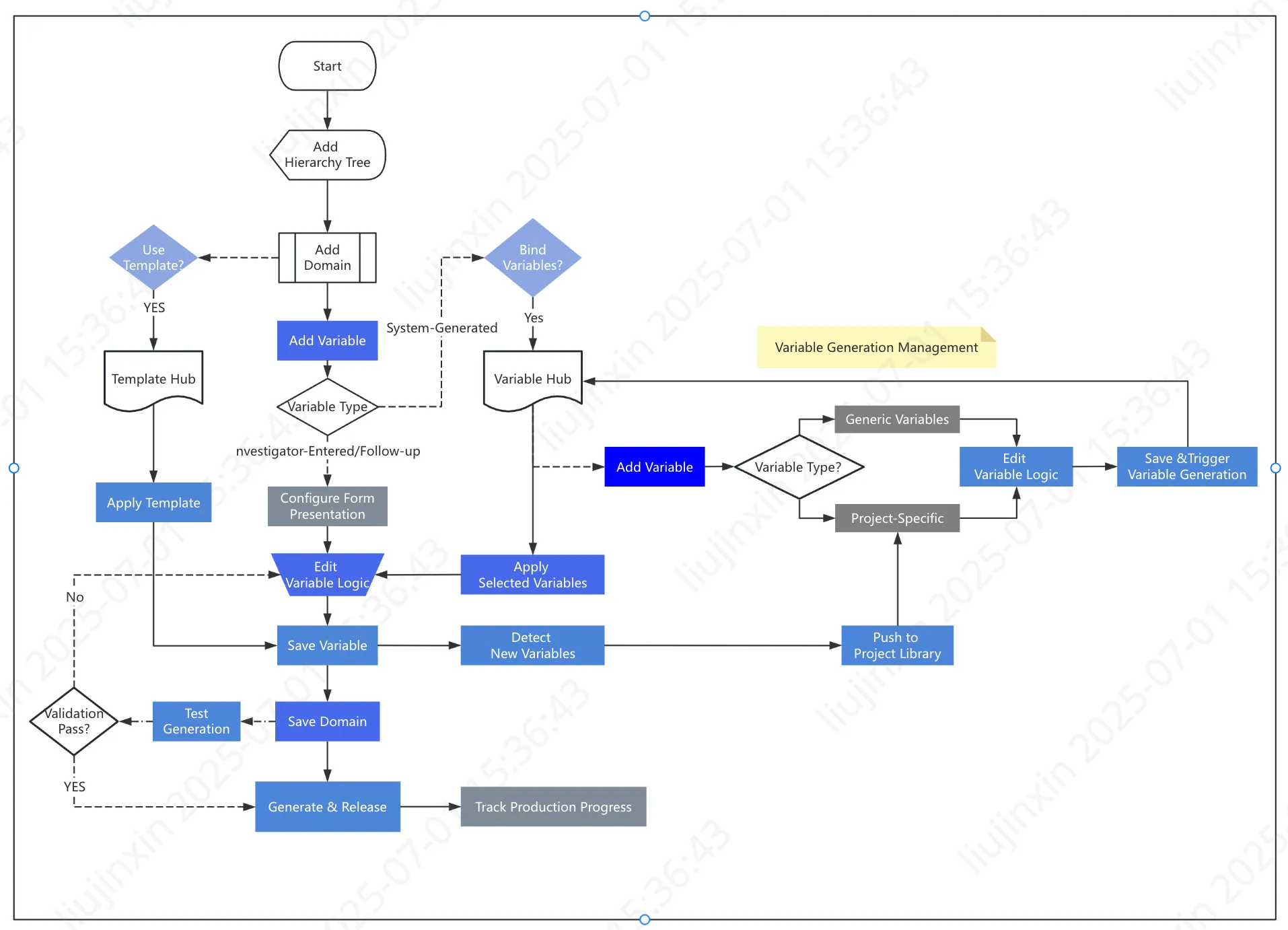
Specialty Disease Database: Variable Generation & Application Workflow
(Illustrated using disease-specific variable production logic)
1️⃣ Core Process
Phase 1: Variable Tree & Definition
- Domain Hierarchy Establishment
- Define research-specific data domains (e.g., “Patient Demographics”, “Lab Results”, “Imaging Findings”)
- Implement domain hierarchy tree for intuitive navigation (300+ domains across studies)
Phase 2: Variable Production
- Variable Creation Methods
- Template-based variables: Reuse existing domain templates
- Rule-based variables: Create custom logic for value derivation
- Manual-entry variables: Enable auto-fill with value binding
- CRF follow-up special handling:
- Configure tiered attributes (follow-up schedules, mobile push)
- Embed verification identifiers
Variable Release Management
- Monitor production status (project-specific/generic variables)
- Edit logic & execute test runs
Core Design Principle → Maximize reuse of existing variables/templates to:
- Streamline optimization cycles
- Enhance universality and usability
2️⃣ Key Innovations
Low-Cost Reusability
- Unified framework for form/variable/logic reuse
- Reduces implementation redundancy by ≈70%
Medical Workflow Integration
- Real-time adjustment of:
- Data hierarchies
- Display formats → Generates verification-ready collection forms
- Real-time adjustment of:
Pre-release Testing
- Controlled production via:
- Pre-deployment variable validation
- Server resource optimization
- Prevents data corruption from untested logic
- Controlled production via:
Incremental Update Engine
Update Type Functionality Full Regeneration Rebuilds all historical data Incremental Updates Processes only new/modified records Overwrite Selective field replacement - Subscription mechanism:
- Push notifications for production status
- Third-system API integration
- Subscription mechanism:
Project Workflow
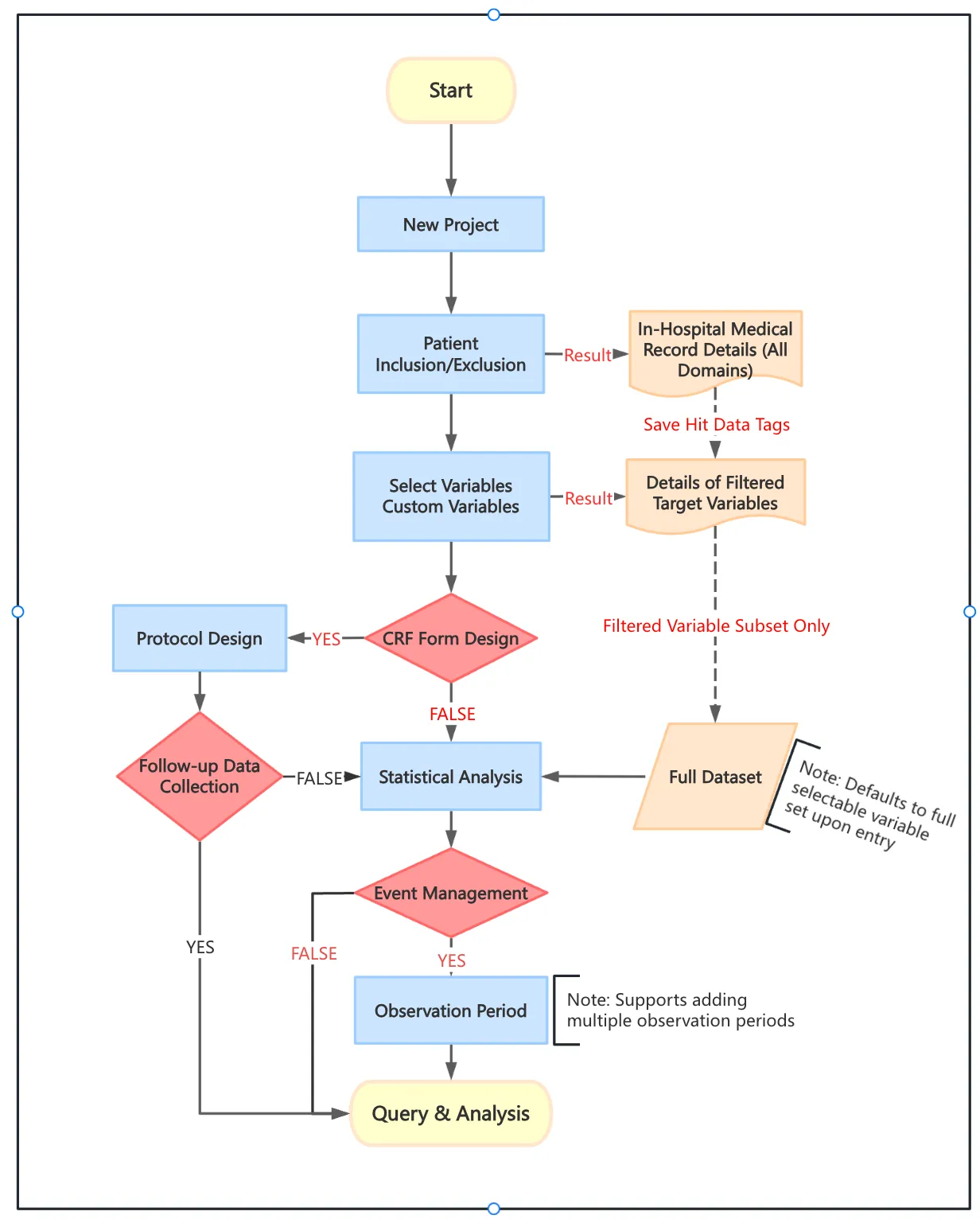
I. Core Process
Project Creation & Cohort Definition
- Set inclusion/exclusion criteria
- Track patient/variable matches with persistent hit flags
Variable Selection
- Choose predefined variables or create custom ones:
- Define logic rules
- Configure aggregation methods
- Specify variable types
- Choose predefined variables or create custom ones:
CRF Form Configuration
- Integrate multi-source data:
- Manual entry fields
- Custom domains
- Follow-up data collection → Forms serve as statistical analysis-ready metadata
- Integrate multi-source data:
Observation Windows (Event Management)
- Create time-bound events when standard variables are insufficient
- Key features:
- Customizable offset windows (pre/post-event)
- Precision value aggregation within windows
Derived Variables
- Combine variables/events with:
- Event-triggered computation
- Custom aggregation rules
- System capabilities:
- Subscription-based auto-calculation
- Manual triggering
- Visual derivation tracing
- Combine variables/events with:
II. Breakthrough Innovations
Unified Workflow Engine
- Shared logic across:
- Projects
- Search result datasets
- Specialty databases
- Consistent handling of:
- Raw vs. matched data
- Variable reuse
- Analytical computations
- Shared logic across:
Dynamic Observation Windows
- Enables:
- Event-based searching
- Low-cost complex variable generation
- First platform to implement temporal variable binding
- Enables:
Visual Variable Derivation
- Workflow components provide:
- Step-by-step generation visibility
- Test execution interfaces
- Full audit trail of logic flows
- Workflow components provide:
Multi-version Dataset History
Layer Function Historical snapshots Enable reproducible analysis Immutable versions Support group comparisons Cleansed baselines Feed reporting pipelines
Note:Key Terminology
| Chinese Term | English Equivalent | Technical Context |
|---|---|---|
| 纳排条件 | Inclusion/Exclusion Criteria | Clinical research |
| 命中标识 | Hit Flags | Data matching |
| 事件管理 | Event Management | Temporal analytics |
| 事件偏移 | Offset Windows | Time-series computation |
| 衍生变量 | Derived Variables | Data modeling |
| 多层历史 | Immutable Versioned Snapshots | Data versioning |
Search & Export Core Features
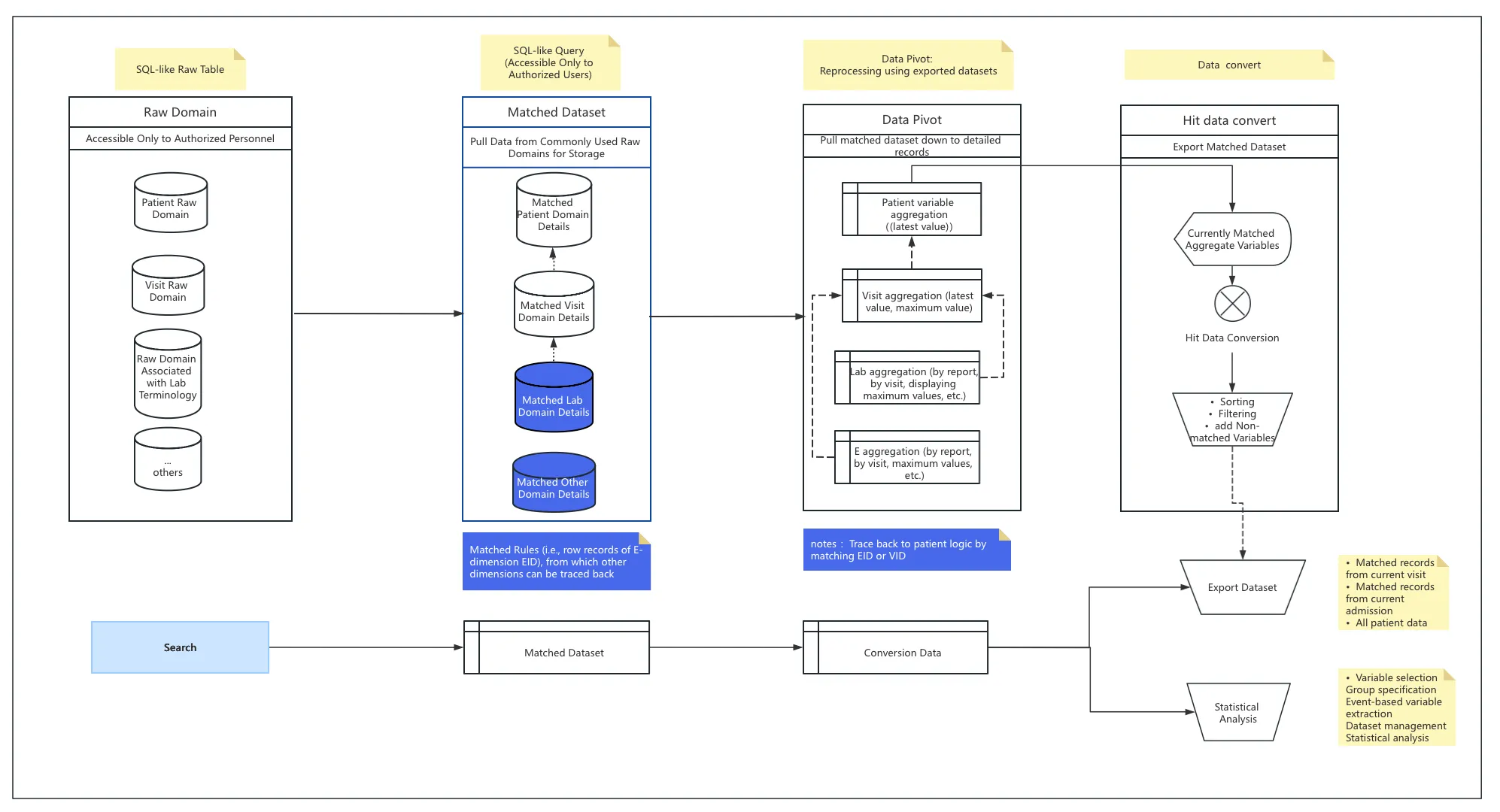
I. Key Capabilities
Entity-Level Precision Search
- Executes variable-based queries directly on source databases
- Returns granular row-level matches (E-dimension precision)
- Enables:
- Targeted exports
- High-accuracy analysis
- Efficient value transformation
Multi-Dimensional Export Framework Example: Query “WBC > 2x upper limit” generates:
Export Option 1: Raw matched records
Export Option 2: Computed results
Traceability backbone:
flowchart LR A[Matched EID] --> B[Lab Report] B --> C[Encounter ID] C --> D[Co-located Test Metrics]Preserves:
- Entity IDs (EID)
- Computation logic IDs
- Cross-document provenance
Temporal Observation Window
- Implements event-anchored searching:
- Customizable pre/post-event offsets
- Multi-layer aggregation modes
- Foundation for:
- AI-powered NLQ (Natural Language Query)
- Contextual clinical exploration
- Implements event-anchored searching:
II. Breakthrough Architecture
| Feature | Innovation | Impact |
|---|---|---|
| E-Dimension Search | Direct source-level entity resolution | 90% reduced dataset size vs. full-table scans |
| Intelligent Exports | Dual-mode raw/computed output | Eliminates post-export processing |
| Temporal Binding | Event-relative time fencing | Enables longitudinal variable derivation |
| Traceability | Embedded EID/computation IDs | Full audit trail from result → source report |
Notes:Critical Terminology
| Chinese Term | English Equivalent | Technical Context |
|---|---|---|
| E维度 | Entity-Level Dimension | Data granularity |
| 命中行记录 | Row-Level Matches | Query execution |
| 预算 | Computation | Data transformation |
| 观测窗 | Temporal Observation Window | Time-series analytics |
| 事件点 | Anchor Event | Temporal reference |
| 溯源 | Provenance Tracing | Data lineage |
| 自然语言对话搜索 | NLQ (Natural Language Query) | AI search interface |